What is Spectroscopy?
Spectroscopy is the study of colour and light. Every colour we see is a result of light being emitted or reflected by atoms. More specifically, it all comes down to electrons, the tiny, charged particles that atom. When these electrons absorb energy, they get “excited” and jump to a higher energy level. But they can’t stay at a higher energy state for long. When they fall back to their original level, they release the excess energy as a photon which is a small burst of light.
The amount of energy released determines the colour of that light. A small drop in energy might release a low-energy photon that would appear red to our eyes. A bigger drop in energy gives off a higher energy photon that might look blue or violet, or even ultraviolet (and thus invisible). This process of electrons converting energy into photons is called atomic emission.
Each Element Has Its Own Colour Signature
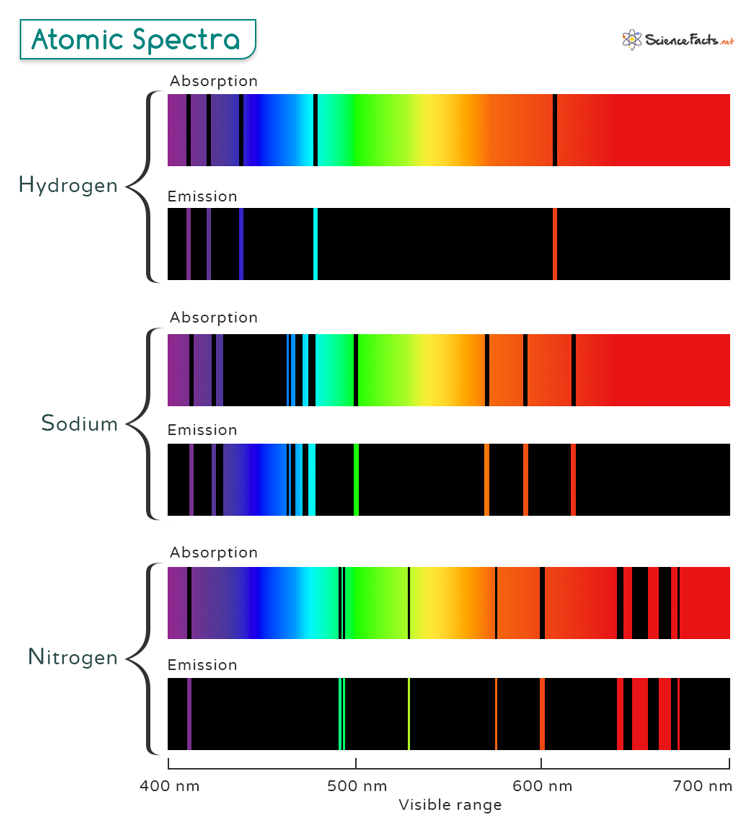
Every type of atom has a unique structure. This means each element emits photons of specific energies — and therefore specific colours when its electrons are energised. When we pass this light through a prism or special filter, we can split it into a spectrum of colours, a bit like a rainbow. This spectrum acts like a barcode for the element: hydrogen has one pattern, oxygen another, sodium another still. These are called atomic spectra.
Looking Into the Stars
Astronomers use atomic spectra when studying stars, galaxies and planets. They are able to look at the light these things emit (and don’t emit) and tell what elements they are composed of.
This technique called spectroscopy has helped us learn that stars like our sun are mostly hydrogen and helium and that distant galaxies contain the same elements we find here on Earth. All of this knowledge comes from watching how energised electrons emit photon, these tiny carriers of information.
Why Spectroscopy Matters for Our Children
For children learning science, it’s powerful to understand that light is something we can read. It carries clues about the universe, about life, and even about their own bodies. Melanin, and specifically eumelanin absorbs and interacts with photons across a wide range of energies. This means that our very bodies are deeply connected to light. And I agree with those who suggest that light is actually the primary means the universe uses to communicate with itself!